so2 lewis dot|SO2 (Sulfur Dioxide) Lewis Structure : iloilo This chemistry video tutorial explains how to draw the lewis structure of SO2 also known as Sulfur Dioxide. It discusses the molecular geometry, bond angle, hybridization and formal charges of. Enjoy your weekends with us.🏖️🌅🌈🥂 💯💦🍜🥳Venue - Opp. Bhilwara Saras Dairy, Ajmer road, Bhilwara RajasthanContact- 7357219650Follow @royals_club1 Map .
PH0 · Sulfur dioxide (SO2) Lewis Structure, Hybridization
PH1 · Sulfor dioxide: Lewis dot structure for SO2 (video)
PH2 · SO2(Sulfur Dioxide) Lewis Structure, Hybridization, Molecular
PH3 · SO2 Lewis Structure: How to Draw the Dot Structure for S02
PH4 · SO2 Lewis Structure, Hybridization, Molecular
PH5 · SO2 Lewis Structure
PH6 · SO2 (Sulfur Dioxide) Lewis Structure
PH7 · Lewis Structure of SO2 (sulfur dioxide)
PH8 · Lewis Dot of Sulfur Dioxide SO2
Watch San Mig Light Para sa Mahaba-habang Kantutan Este Usapan 2 on Pinayflixtv.net, the hottest Pinay porn site. PinayFlix has the best sex Model, Teen collections of videos for free.
so2 lewis dot*******Learn how to draw the dot structure for sulfur dioxide (SO2) with or without resonance structures. See the video transcript, questions and tips from other learners.
How to draw the Lewis Structure of SO2 - with explanationCheck me out: http://www.chemistnate.com A step-by-step explanation of how to draw the SO2 Lewis Structure (Sulfur Dioxide) Note: From an experimental view (using x-ray crystallography or something similar) we find the . This chemistry video tutorial explains how to draw the lewis structure of SO2 also known as Sulfur Dioxide. It discusses the molecular geometry, bond angle, hybridization and formal charges of.
Learn how to draw the Lewis structure of SO2, a colorless gas with a pungent odor, and its resonance forms. Find out its hybridization, molecular geometry, bo.
Learn how to draw the lewis structure of SO2, a pungent-smelling gas with various industrial uses. Find out its hybridization, molecular geometry, and MO diagram using the formula and theory.Learn how to draw the Lewis structure for SO2, a molecule with a central sulfur atom and two oxygen atoms. Follow the steps to count valence electrons, identify the central atom, connect the atoms, distribute the .The Lewis structure for SO 2 requires you to place more than 8 valence electrons on Sulfur (S). You might think you've got the correct Lewis structure for SO 2 at first. Remember, .Sulfur having valence electrons in the 3rd energy level, would also have access to the 3d sublevel, thus allowing for more than 8 electrons. NOT IN THIS MOLECULE. Sulfur .In SO2 lewis structure, there are two double bonds between sulfur atom and oxygen atoms. Sulfur dioxide lewis structure is drawn step by step using VESPR rules. S and O atoms have sp2 hybridization.SO2 (Sulfur Dioxide) Lewis StructureSO2 Lewis Structure Step-by-Step Guide. To draw the SO2 Lewis structure, follow these simple steps: 1. Determine the total valence electrons. Start by counting the valence electrons of each atom in the .SO 2. Back. 70 More Lewis Dot Structures. S does not follow the octet rule. It can hold more than 8 electrons. Sulfur having valence electrons in the 3rd energy level, would also have access to the 3d sublevel, thus allowing for more than 8 electrons. NOT IN THIS MOLECULE. Sulfur dioxide emissions are a precursor to acid rain and atmospheric . Today in this video, we will determine the Lewis dot structure for sulphur dioxide, having a chemical formula of SO2. It comprises one sulphur atom and two O.
A video explanation of how to draw the Lewis Dot Structure for Sulfur Dioxide, along with information about the compound including Formal Charges, Polarity, . SO2 lewis structure of total valence electrons 18.Sulfur and oxygen has six electrons. sulfur has six valence electrons, 2 non bonding and 6 bonding electrons. Six bonding electrons divided by 2 , we get 3 electrons. So the Formal charge of sulfur is 6-2-3 =+1. One of the oxygen having formal charge +1.
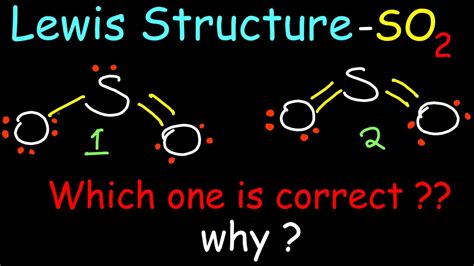
The SO2 Lewis structure depicts the molecular arrangement of sulfur dioxide, which consists of one sulfur atom and two oxygen atoms. In the SO 2 Lewis structure, there is a double bond between the sulfur atom and each oxygen atom. Each oxygen atom possesses two lone pairs, while the sulfur atom has one lone pair. The SO2 Lewis structure depicts the molecular arrangement of sulfur dioxide, which consists of one sulfur atom and two oxygen atoms. In the SO 2 Lewis structure, there is a double bond between the sulfur atom and each oxygen atom. Each oxygen atom possesses two lone pairs, while the sulfur atom has one lone pair.Drawing the Lewis Structure for SO 2. The Lewis structure for SO 2 requires you to place more than 8 valence electrons on Sulfur (S). You might think you've got the correct Lewis structure for SO 2 at first. Remember, Sulfur is in Period 3 and can hold more than 8 valence electrons. You'll want to calculate the formal charges on each atom to . Here are the steps I follow when drawing a Lewis structure. > 1. Decide which is the central atom in the structure. That will normally be the least electronegative atom ("S"). 2. Draw a skeleton structure in which the other atoms are single-bonded to the central atom: "O-S-O". 3. Draw a trial structure by putting electron pairs around every .Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.so2 lewis dotThe Lewis Dot Structure for SO 2:. Sulfur dioxide (SO 2) is a gas which can be toxic, is a constituent of volcanic gasses, and is produced by some fossil burning activities.To better understand the nature of the chemical bonding in the molecule, a Lewis structure can be drawn to analyze how the valence electrons are used to make the bonds.
Using the cross bow arrow shown below we can show that it has a net dipole. The net dipole is the measurable, which is called the dipole moment. Dipole moment is equal to the product of the partial charge and the distance. The equation for dipole moment is as follows. μ = δ × d (3.7.1) (3.7.1) μ = δ × d. with.

Contributors and Attributions. 9.4: Resonance Lewis Structures is shared under a license and was authored, remixed, and/or curated by LibreTexts. Resonance is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by a single Lewis formula.so2 lewis dot SO2 (Sulfur Dioxide) Lewis Structure Contributors and Attributions. 9.4: Resonance Lewis Structures is shared under a license and was authored, remixed, and/or curated by LibreTexts. Resonance is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by a single Lewis formula.
The formal charges of the SO 2 with the single bond and a double bond is larger than the SO 2 with two double bonds. So I would assume that the one with two double bonds is the correct structure. But chemistry books . An explanation of the molecular geometry for the SO2 ion (Sulfur dioxide) including a description of the SO2 bond angles. The electron geometry for the Sulfu. The SO2 Lewis structure would consist of two oxygen (O) atoms and one sulfur atom. Both the sulfur and oxygen atoms have six valence electrons. The molecular geometry of sulfur dioxide is a bent shape. The sulfur to oxygen ratio in sulfur dioxide is 1:2. The sulfur dioxide molecule has two double bonds between the Sulfur atom and Oxygen . A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and . SO2 lewis structure has a Sulfur atom (S) at the center which is surrounded by two Oxygen atoms (O). There are 2 double bonds between the Sulfur atom (S) and each Oxygen atom (O). . In the above lewis dot structure of SO2, you can also represent each bonding electron pair (:) as a single bond (|). By doing so, you will get the following lewis .
This page titled 9.2: Lewis Electron Dot Diagrams is shared under a license and was authored, remixed, and/or curated by via that was edited to the style and standards of the LibreTexts platform. Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. Lewis electron dot diagrams for ions have less (for .
STL- Camarines SurWelcome to the SportyBet App, the ultimate destination for a thrilling sports betting experience. Explore our wide range of sports betting options, with a special focus on football. Enjoy diverse markets, unbeatable odds, enticing bonuses, an in-house casino for added excitement, responsible gamblin.
so2 lewis dot|SO2 (Sulfur Dioxide) Lewis Structure